
AlphaFold and the Solution to Levinthal’s Paradox: AI’s Greatest Scientific Breakthrough
When we talk about the most important contributions of artificial intelligence, most people immediately think of large language models or stock-prediction systems. But AlphaFold and the solution to Levinthal’s Paradox represent something far more profound. They mark the moment AI shifted from beating humans at board games to solving a billion-dollar scientific problem that experts previously believed unsolvable. This was the turning point where AI proved it could reason about the physical world, not just text.

What Was Considered Unsolvable: Levinthal’s Paradox
Predicting the shape of a protein was long considered one of biology’s hardest challenges. A protein chain contains hundreds of amino acids (“beads”), each capable of rotating in many directions. The number of possible shapes a single protein could form is mind-bending—estimated at 10^300. For comparison, the observable universe contains only about 10^80 atoms.
Yet in nature, proteins fold correctly in milliseconds.
This contradiction is known as Levinthal’s Paradox, and solving it was believed to be decades away—until AI arrived.
The Old Way: Freezing Time to Capture Protein Structure
Before AlphaFold and the solution to Levinthal’s Paradox, scientists used costly and time-consuming techniques to “catch” proteins in their final folded shape.
X-Ray Crystallography
Researchers first had to crystalize the protein. Once crystallized, high-powered X-rays were fired at it, creating diffraction patterns that could be interpreted mathematically to infer the protein’s structure.
Cryo-Electron Microscopy (Cryo-EM)
Alternatively, scientists flash-froze proteins in liquid ethane so rapidly that water didn’t form crystals. Thousands of blurred images were averaged to reconstruct a 3D structure.
Even with luck, these methods could take 1–5 years and cost $100,000+ per protein.
How AI Changed Everything
Instead of treating protein folding as a brute-force calculation, AlphaFold treats it as a pattern-recognition puzzle, learning from the evolutionary history encoded in related proteins.
If a human protein shares similarities with proteins in mice, fish, or bacteria, the AI identifies conserved patterns. For example, if amino acid #5 changes charge and amino acid #67 simultaneously changes in a coordinated way across species, AlphaFold infers that these two are likely close in 3D space.
This evolutionary insight is the foundation of its power.
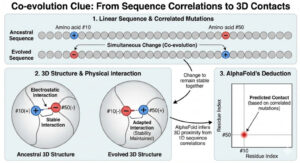
Under the Hood: The Evoformer Architecture
At the core of AlphaFold and the solution to Levinthal’s Paradox is a specialized neural network called the Evoformer, developed by Google DeepMind.
Track A — The Sequence View (1D)
The model examines the amino acid chain as a linear sequence.
Track B — The Pair Representation (2D)
A matrix maps potential distances between every pair of amino acids, generating a probability landscape of physical interactions.
AlphaFold uses attention mechanisms, similar to those in ChatGPT, to detect long-range interactions across the chain. Once these relationships are mapped, the Structure Module converts them into 3D coordinates, treating the protein backbone like a set of “floating triangles” that rotate and snap into place under geometric constraints.
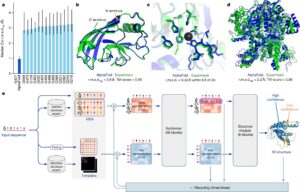
The Impact: Years of Work Reduced to Minutes
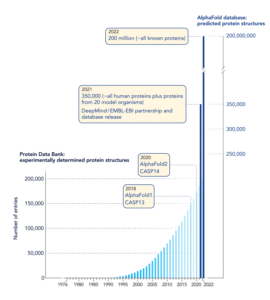
Before AlphaFold, the world had discovered only ~180,000 protein structures—the result of 60 years of work and billions in funding.
After its release in 2021, AlphaFold predicted over 214 million structures, freely available to researchers.
AlphaFold 3 (2024) extended this further, accurately modeling interactions between:
-
Proteins
-
DNA
-
RNA
-
Drug-like molecules
For this groundbreaking work, Demis Hassabis, John Jumper, and David Baker received the Nobel Prize in Chemistry in 2024.
Why It Matters for Medicine and the Planet
For most of human history, biology has been non-digital. Most modern drugs work by binding to specific protein pockets—pockets we often couldn’t see until now.
Thanks to AlphaFold and the solution to Levinthal’s Paradox, we can now:
-
identify once “undruggable” proteins
-
design bespoke drugs for rare genetic diseases
-
respond to rapidly evolving pathogens
-
engineer enzymes to break down PET plastic
-
design proteins to capture carbon dioxide more effectively than plants
This is the beginning of a true Green Revolution 2.0, powered by synthetic biology.
The Risks: When Power Outpaces Regulation
Not all consequences are positive. Biological data is simple text—easily downloaded, shared, or misused.
Dual-Use Concerns
The same tools that design cures can also design harmful proteins or super-toxins.
Ecological Risk
A well-intentioned engineered microbe designed to digest plastic might escape and attack unintended materials—like electrical insulation or vehicle components.
Regulating AI-driven biology may be even harder than regulating nuclear technology.
Conclusion: The Dawn of Digital Biology
The emergence of AlphaFold and the solution to Levinthal’s Paradox is comparable to the invention of plastic—transformative, world-changing, and carrying massive responsibility.
For decades we wondered whether AI could solve real scientific problems. AlphaFold answered that question. It demonstrated that AI can decode the physical world and reveal the hidden structure of life.
We now possess the “source code” of biology.
What we choose to build with it will define the next century.
Know more about relevant topics –>
AI Assistant CRM: https://codesmiths.in/ai-assistant-for-crm/
Top 3 AI Myths Debunked: https://codesmiths.in/ai-myths-debunked/
AI Powered Disease Surveillance System For Vector-Borne Illness: https://codesmiths.in/ai-powered-disease-surveillance-system-for-vector-borne-illnesses/
AI Powered Social Media Task Automation and Management System: https://codesmiths.in/ai-powered-social-media-task-automation-management-system/



